SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1
By A Mystery Man Writer
Last updated 16 Sept 2024

VIDEO ANSWER: Hello students: let's look at the question: l n, that integrate integration and 0 z minus 1 bracket, close d p by p here. Minus 1 is equal to minus 8.50 into 10 to the power minus 3 p by p, not plus 3.50 into 10. To the power minus 9. P
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Solved NAME: 1.(a) Plot compression factor Z verses pressure

At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as p = dfrac {RT}{V - b}
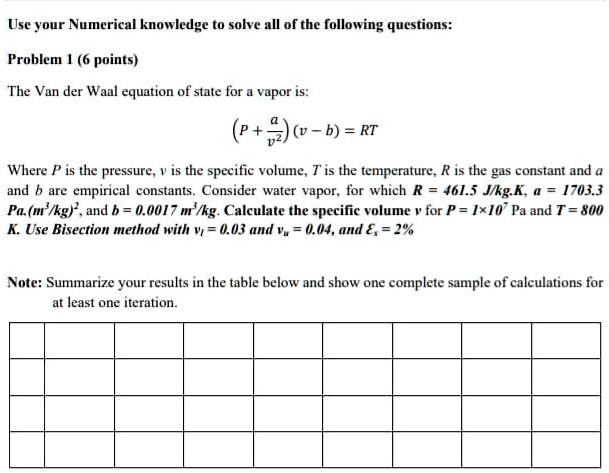
SOLVED: Use our numerical knowledge to solve all of the following questions: Problem (6 points) The Van der Waals equation of state for vapor is: (P + a/v^2)(v - b) = RT

Thermodynamics of Physical and Chemical Transformations

Evaluation de la porosité de drainage à partir de limnigrammes de nappes

Solved The compression factor is given by Z = pV/RT = 1 +

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Statistical Physics: a jogging course - Yoono.org
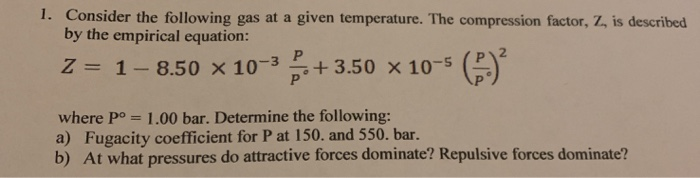
Solved 1. Consider the following gas at a given temperature.

PDF) Solucionario de Física II Para Ciencias e Ingeniería, 7a ed

OneClass: For a gas at a given temperature, the compression factor is described by the empirical equa

Ficoquimica, PDF, Gases

Solved 1) The compression factor, Z, can be written as: Z =
Recommended for you
 Solved 2. By definition, the compression factor of an ideal14 Jul 2023
Solved 2. By definition, the compression factor of an ideal14 Jul 2023- Solved 9 Compression factor Z Use the van-der-Waals equation14 Jul 2023
 Answered: Compression factor of a gas with van…14 Jul 2023
Answered: Compression factor of a gas with van…14 Jul 2023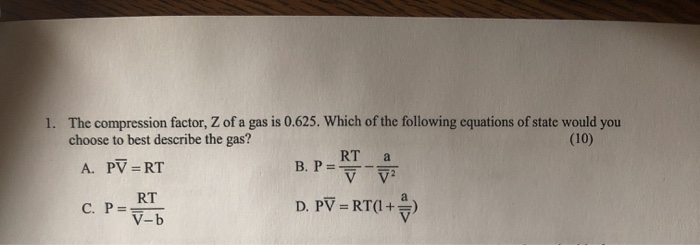 Solved 1. The compression factor, Z of a gas is 0.625. Which14 Jul 2023
Solved 1. The compression factor, Z of a gas is 0.625. Which14 Jul 2023 a) Suppose that $10.0\ \mathrm{mol}\ \mathrm{C}_{2} \mathrm14 Jul 2023
a) Suppose that $10.0\ \mathrm{mol}\ \mathrm{C}_{2} \mathrm14 Jul 2023 Show that the van der Waals equation leads to values of Z <14 Jul 2023
Show that the van der Waals equation leads to values of Z <14 Jul 2023- Answer in General Chemistry for Carl #27553314 Jul 2023
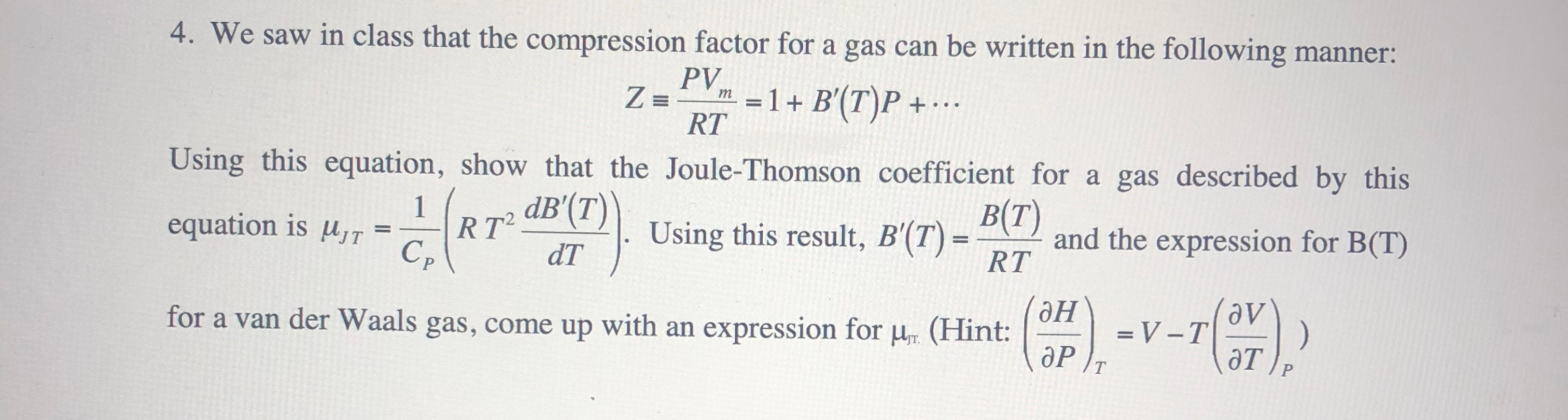 Solved Z = 4. We saw in class that the compression factor14 Jul 2023
Solved Z = 4. We saw in class that the compression factor14 Jul 2023 Non-ideal behavior of gases (article)14 Jul 2023
Non-ideal behavior of gases (article)14 Jul 2023 the compression factor one mole of a vander waals gas 0 C and 10014 Jul 2023
the compression factor one mole of a vander waals gas 0 C and 10014 Jul 2023
You may also like
 Old Navy High-Waisted PowerSoft 7/8 Joggers for Women beige - 61349131214 Jul 2023
Old Navy High-Waisted PowerSoft 7/8 Joggers for Women beige - 61349131214 Jul 2023 Cute Jeans – ShopVani14 Jul 2023
Cute Jeans – ShopVani14 Jul 2023 Presence Sweatshirt14 Jul 2023
Presence Sweatshirt14 Jul 2023 Three Sided Brass Bristle BBQ Grill Cleaning Brush for Cast Iron Grids with Integrated Bottle Opener14 Jul 2023
Three Sided Brass Bristle BBQ Grill Cleaning Brush for Cast Iron Grids with Integrated Bottle Opener14 Jul 2023 Under Armour Charged Escape Mens 8 Sneakers Shoes Running Black 3020004-00114 Jul 2023
Under Armour Charged Escape Mens 8 Sneakers Shoes Running Black 3020004-00114 Jul 2023 Bigersell Training Bra Women Beautiful Comfortable Backless With Shoulder Straps Sports Bras Everyday Bras Women Size Full Coverage Bra, Style 1016714 Jul 2023
Bigersell Training Bra Women Beautiful Comfortable Backless With Shoulder Straps Sports Bras Everyday Bras Women Size Full Coverage Bra, Style 1016714 Jul 2023 Royal Blue Formal Pantsuit Women, Three Piece Pantsuit, Single14 Jul 2023
Royal Blue Formal Pantsuit Women, Three Piece Pantsuit, Single14 Jul 2023- b.tempt'd b.adorable Lace-Trim Bralette 935182 - Macy's14 Jul 2023
 King Fit Boxers - Black – Box Menswear14 Jul 2023
King Fit Boxers - Black – Box Menswear14 Jul 2023 Buy Wear Everywhere T-Shirt Lightly Lined Bra Online14 Jul 2023
Buy Wear Everywhere T-Shirt Lightly Lined Bra Online14 Jul 2023

